The growth of Duchenne Muscular Dystrophy (DMD) Market is anticipated to increase because of an increasing incidence of DMD patients as well as the launch of emerging drugs in the 7MM.
DelveInsight has introduced a new Market Insights, Epidemiology, and Market Forecast Report on “Duchenne Muscular Dystrophy Market Insights, Epidemiology and Market Forecast-2030” to its portfolio.
Key Highlights from the report are:
- As per the DelveInsight analysis, the total diagnosed Duchenne Muscular Dystrophy prevalent population in the 7 MM was 27,685 in 2017.
- The total diagnosed prevalent cases of DMD patients were found to be maximum in the age-group of 8–13 year and 14–19 year in the United States in 2017.
- The treatment for DMD patients is the standard care along with the new upcoming therapeutic strategies covering Genetic Therapies, Cell therapy using muscle precursor cells or stem cells, Membrane stabilization and upregulation of cytoskeletal proteins and secondary cascades treatment.
- Duchenne Muscular Dystrophy market size in the 7MM is expected to increase during the study period. As per the estimates, the highest contribution in the market size of DMD is from the United States, followed by EU-5 countries and Japan.
Duchenne Muscular Dystrophy is a progressive form of muscular dystrophy, which usually occurs in males, though in rare cases may affect females as well, causing progressive weakness and loss (atrophy) of skeletal and heart muscles.
The disease epidemiology covered in the report proffers historical as well as forecasted Duchenne Muscular Dystrophy epidemiology, which is segmented as Diagnosed Prevalent Population of DMD, Age-specific Diagnosed Prevalence of DMD, Mutation-specific Diagnosed Prevalence of DMD and Diagnosed Prevalence of Associated Comorbidities in DMD in the 7MM market from 2017 to 2030.
The report also covers Mutation-specific Diagnosed Prevalence of Duchenne Muscular Dystrophy, including several mutations such as Large Mutations, Small Mutations and Point Mutations with major proportion for deletions in Large Mutations subgroup.
Duchenne Muscular Dystrophy report encloses the detailed analysis of DMD marketed drugs and mid and late-stage pipeline drugs.
The therapies that are approved for the DMD treatment are Vyondys 53 (Golodirsen), Emflaza, Exondys 51, Translarna along with many more.
Drugs covered in the report are:-
There are several key players robustly involved in developing potential products such as
- Casimersen
- SRP-9001
- Puldysa (Idebenone)
- Givinostat
- Edasalonexent
- Viltolarsen
- PF-06939926
- Vamorolone
- TAS-205
- Pamrevlumab (FG-3019)
- Allogeneic Cardiosphere-Derived Cells (CAP-1002)
- DS-5141b
And many others
Key Players covered in the DMD market report are:-
- Sarepta Therapeutics
- Italfarmaco
- Catabasis Pharmaceuticals
- Nippon Shinyaku (NS Pharma)
- Pfizer
- Santhera Pharmaceuticals/ReveraGen BioPharma
- Taiho Pharmaceutical
- FibroGen
- Capricor
- Daiichi Sankyo
And many others
The reasons for buying this report:
- The report proffers an overview of pathophysiology, various diagnostic approaches and Duchenne Muscular Dystrophy treatment algorithm, including detailed chapters for marketed products and emerging therapies.
- Historical and forecasted DMD epidemiology in 7MM covering the United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom), and Japan from 2017-2030.
- Detailed historical and forecasted Duchenne Muscular Dystrophy market covering the United States, EU5 and Japan from 2017-2030.
- Pipeline analysis across different stages of development (Phase III and Phase II), different emerging trends and comparative analysis of pipeline products with comprehensive clinical profiles, key cross-competition, launch date along with product development activities.
- Detailed DMD market size by therapies, covering the United States, EU5 and Japan from 2017-2030.
- Reimbursement scenario and Key Opinion Leader Views.
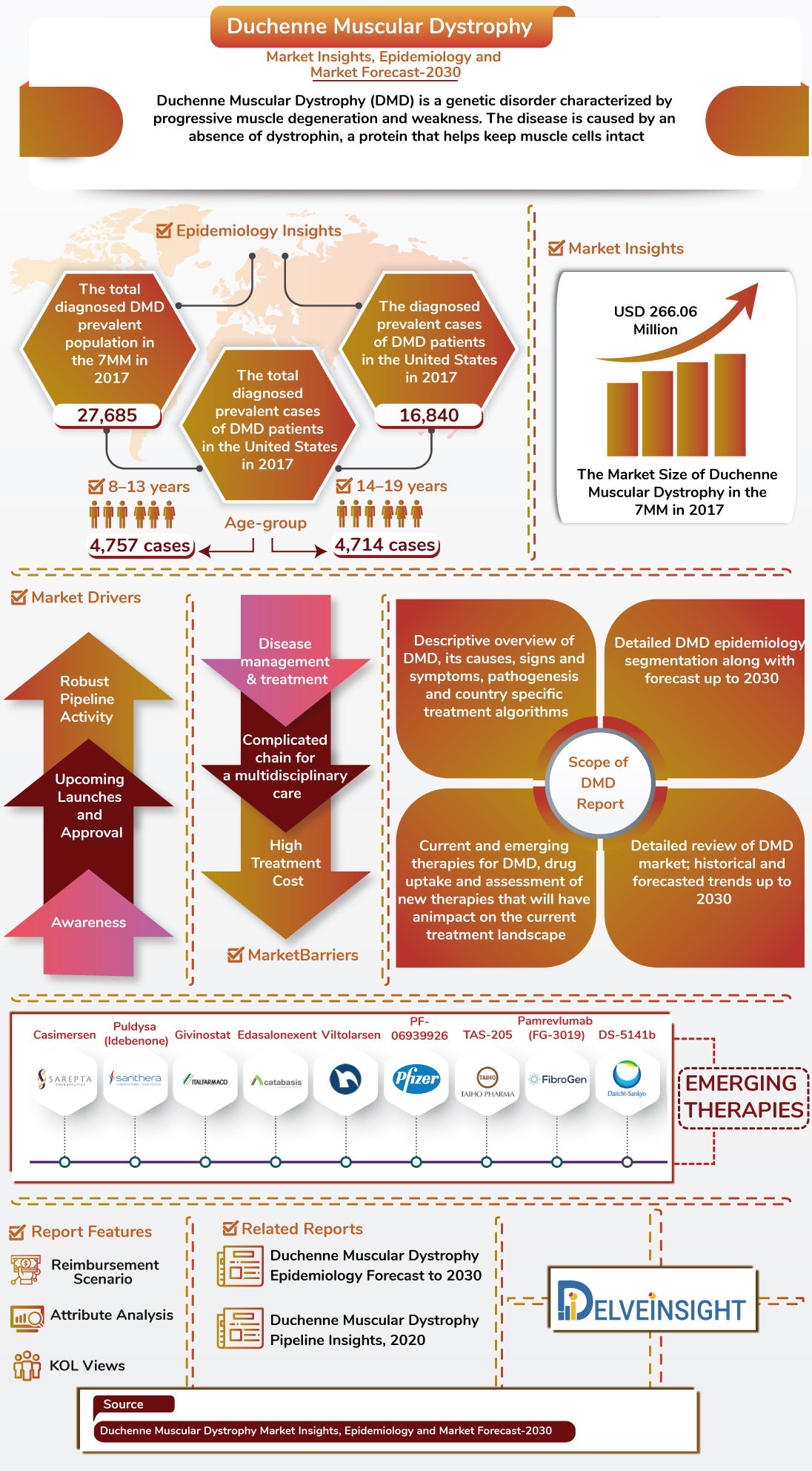 Table of Contents
Table of Contents
|
1 |
Key Insights |
|
2 |
Duchenne Muscular Dystrophy Market Overview at a Glance |
|
3 |
DMD Disease Background and Overview |
|
4 |
Recognized Establishments |
|
5 |
Duchenne Muscular Dystrophy Epidemiology and Patient Population |
|
6 |
Country Wise-Epidemiology of DMD |
|
6.1 |
United States |
|
6.2 |
EU5 Countries |
|
6.3 |
Germany |
|
6.4 |
France |
|
6.5 |
Italy |
|
6.6 |
Spain |
|
6.7 |
United Kingdom |
|
6.8 |
Japan |
|
7 |
Duchenne Muscular Dystrophy Treatment |
|
8 |
General Guidelines on DMD |
|
9 |
United States Guidelines on Duchenne Muscular Dystrophy |
|
10 |
European Guidelines on Duchenne Muscular Dystrophy |
|
11 |
Unmet Needs |
|
12 |
DMD Marketed Drugs |
|
13 |
Duchenne Muscular Dystrophy Emerging Drugs |
|
13.1 |
Key Cross Competition |
|
13.2 |
Casimersen: Sarepta Therapeutics |
|
13.3 |
Puldysa (Idebenone): Santhera Pharmaceuticals |
|
13.4 |
Givinostat: Italfarmaco |
|
13.5 |
Edasalonexent: Catabasis Pharmaceuticals |
|
13.6 |
Viltolarsen: Nippon Shinyaku (NS Pharma) |
|
13.7 |
PF-06939926: Pfizer |
|
13.8 |
Vamorolone: Santhera Pharmaceuticals/ReveraGen BioPharma |
|
13.9 |
TAS-205: Taiho Pharmaceutical |
|
13.10 |
Pamrevlumab (FG-3019): FibroGen |
|
13.11 |
SRP-9001: Sarepta Therapeutics |
|
13.12 |
Allogeneic Cardiosphere-Derived Cells (CAP-1002): Capricor |
|
13.13 |
DS-5141b: Daiichi Sankyo |
|
14 |
Duchenne Muscular Dystrophy 7 Major Market Analysis |
|
15 |
The United States Market Outlook |
|
16 |
EU-5 Countries: Market Outlook |
|
16.1 |
Germany |
|
16.2 |
France |
|
16.3 |
Italy |
|
16.4 |
Spain |
|
16.5 |
United Kingdom |
|
17 |
Japan: Market Outlook |
|
18 |
Case Reports |
|
19 |
Market Drivers |
|
20 |
Market Barriers |
|
21 |
SWOT Analysis for Duchenne Muscular Dystrophy |
|
22 |
Appendix |
|
23 |
DelveInsight Capabilities |
|
24 |
Disclaimer |
|
25 |
About DelveInsight |
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research Firm focused exclusively on life sciences. It supports pharma companies by providing end to end comprehensive solutions to improve their performance.
Related Reports:-
- Duchenne Muscular Dystrophy Epidemiology Forecast to 2030
DelveInsight’s Duchenne Muscular Dystrophy – Epidemiology Forecast 2030 report delivers an in-depth understanding of the disease, historical, and forecasted epidemiology of Duchenne Muscular Dystrophy in the United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom), and Japan. - Duchenne Muscular Dystrophy Pipeline Insights, 2020
Duchenne Muscular Dystrophy Pipeline Insight, 2020 report by DelveInsight outlays comprehensive insights of present clinical development scenario and growth prospects across the Duchenne Muscular Dystrophy market.
https://www.delveinsight.co.uk/blog/immune-thrombocytopenia-itp-epidemiology-forecast/


